Abnormal Uterine Bleeding (AUB) is a prevalent disorder often managed with progestin therapy. Systemic delivery of Levonorgestrel (LNG) can lead to elevated plasma levels, increasing the risk of breast cancer.
Intravaginal Rings (IVRs) provide a localized delivery route, minimizing systemic exposure and improving patient compliance. In this project, IVRs were fabricated from silicone, ethylene-vinyl acetate, and polyurethane containing LNG in both matrix and reservoir formats. Afterwards, they were investigated to find out the desirable LNG release profile.
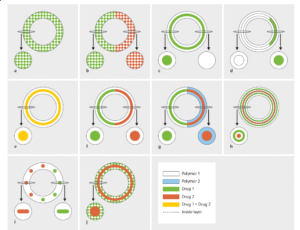
Various types of IVRs were designed, and their drug release performance was studied in silico (as shown in the following figure). Then, the most appropriate designs were fabricated using aluminum laser-cut molds employing different types of polymers.
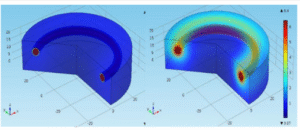
The mechanical properties of the IVRs were tested for tensile strength (stretch without breaking), elongation at break (flexibility), and compression resistance (to mimic vaginal environment).

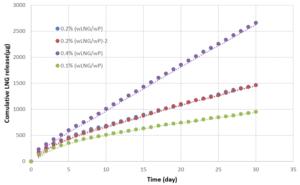
The drug release profile of the four selected formulations were compared based on their cumulative LNG release, showing variation in release kinetics in various materials. IVRs exhibited sustained release over 28 days. Therefore, this new therapeutic method for delivery of LNG can remarkably reduce its systemic side effects and enhance the efficacy of AUB management.
In Press, check back for updates.
