Departments
05 in vitro III (microbiology)
05 in vitro III: Microbial Culture, Product Development & Efficacy Testing
Introduction
At INVITROVO, the 05 in vitro III (microbiology) department is a specialized center for high-quality in vitro testing, focusing on the versatile applications of microorganisms and the safety validation of biomedical materials. We are dedicated to the comprehensive culture of various microorganisms, including microalgae and fungi, and the subsequent evaluation of their byproducts for use in advanced applications like drug delivery systems and tissue engineering. Simultaneously, we conduct microbiological testing to assess the safety, efficacy, and antimicrobial properties of new biomaterials, ensuring they meet therapeutic objectives under clinically relevant conditions. Our experts collaborate closely with the Biomaterial and Cytology Departments, utilizing in vitro protocols designed to replicate physiological environments for reliable in vivo performance predictions, ultimately helping to bring biomedical innovations closer to clinical success
Aim
The strategic aim of the Microbiology Department is to harness the therapeutic potential of microbial resources and to provide rigorous antimicrobial and safety validation for all materials. We achieve this by focusing on:
- To Cultivate and Develop Resources: To successfully culture and develop various microorganisms, including microalgae and fungi, and explore the potential of their byproducts for next-generation biomedical solutions.
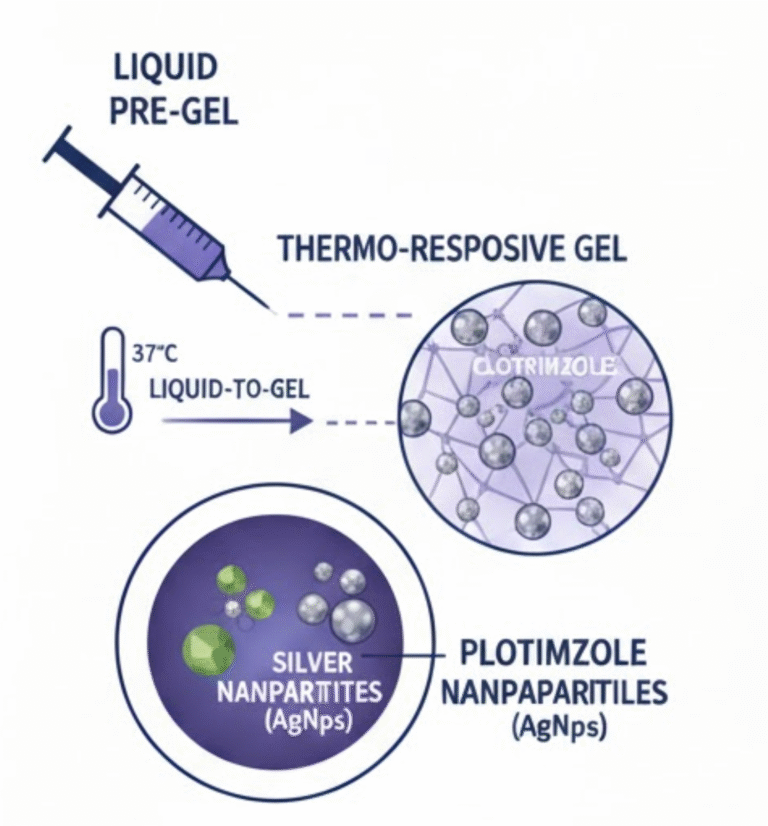
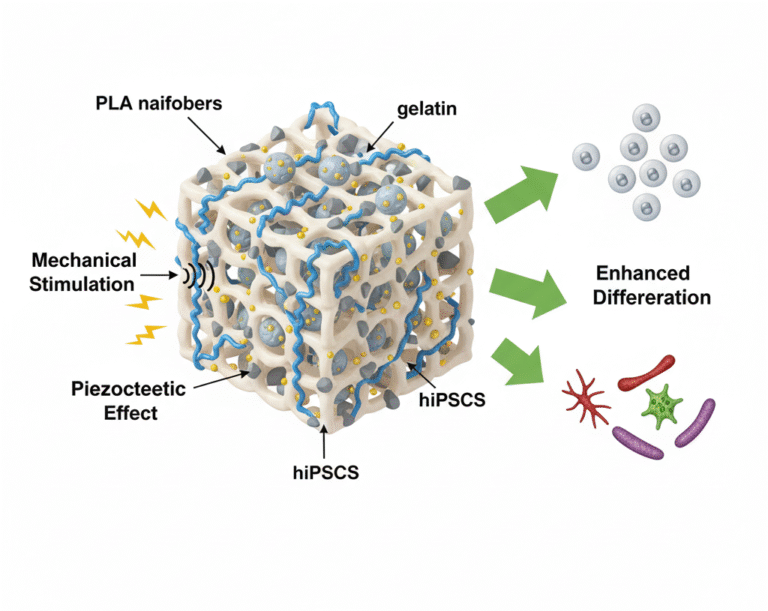
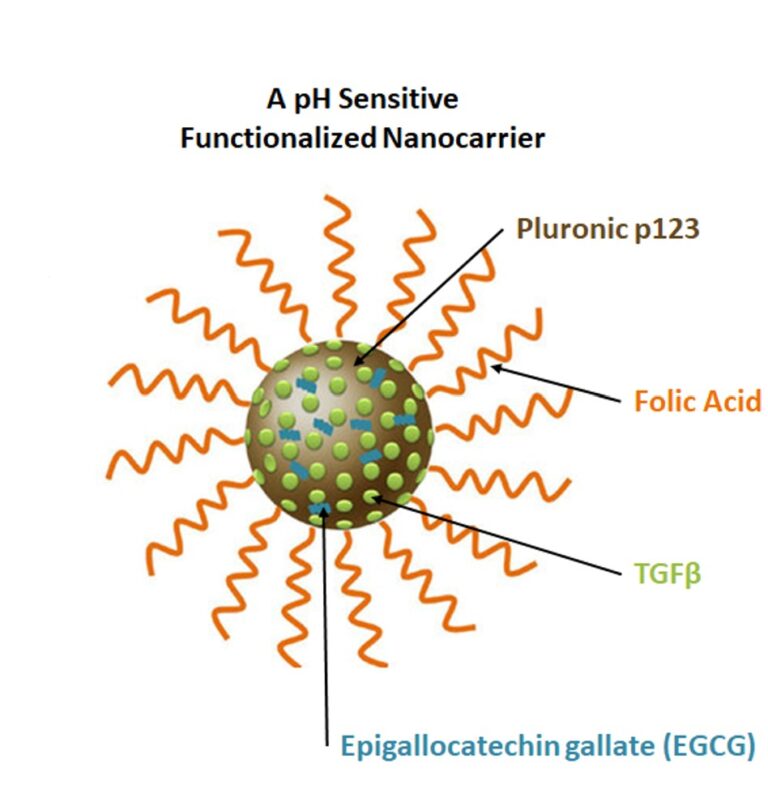
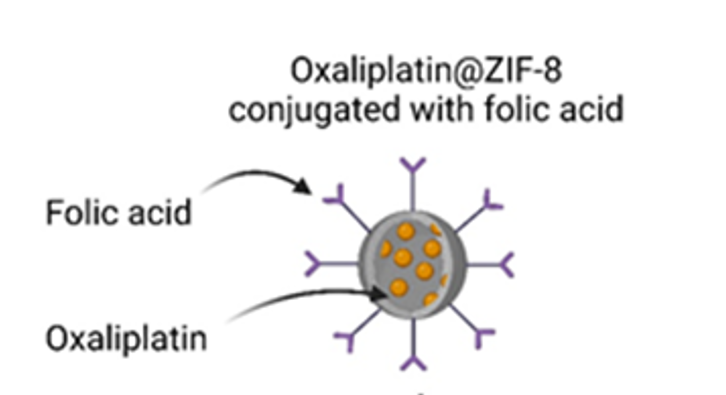
- To Validate Therapeutic Applications: To rigorously test the application of microbial and microalgal byproducts within advanced drug delivery systems and tissue engineering frameworks.
- To Ensure Biological Safety: To conduct rigorous microbiological testing on biomaterials, evaluating their safety, efficacy, and antimicrobial properties to ensure optimal performance under clinically relevant conditions.
Scope
The operational scope of the 05 in vitro III (Microbiology) department encompasses specialized methodologies for both microbial resource development and biological safety validation. We provide comprehensive support covering:
- Microbial Resource Development:
- Culture and propagation of diverse microorganisms, including microalgae and fungi, for use as therapeutic sources in biomedical research.
- Evaluation of microbial and microalgal byproducts for their potential application in drug delivery and tissue engineering.
- Antimicrobial and Safety Testing:
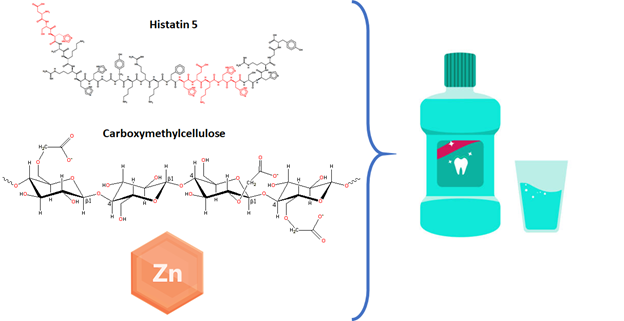
- Comprehensive Efficacy Testing: Performing a wide range of assays to assess the antimicrobial susceptibility, inhibition, and efficacy of biomaterials against various pathogens.
- Biofilm Studies: Investigating the ability of materials to inhibit biofilm formation, a critical factor in implant success and infection control.
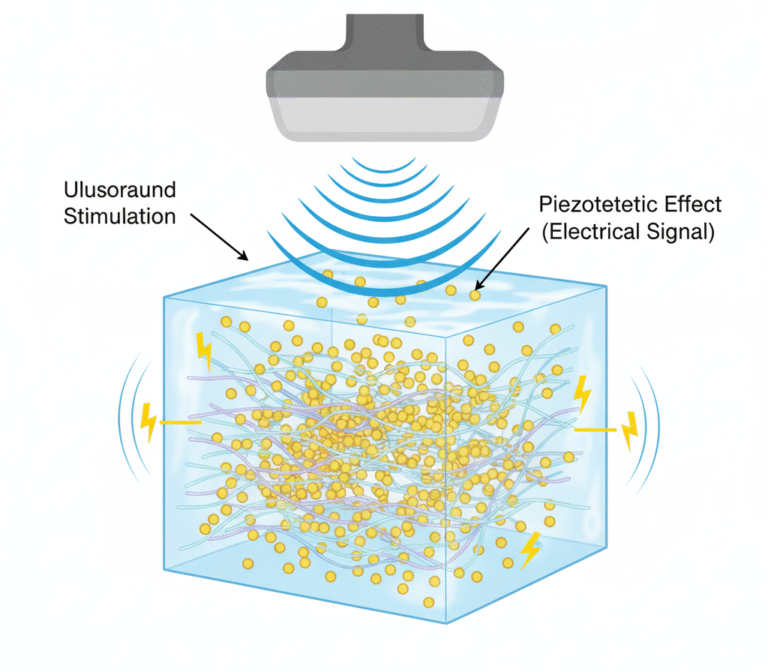
- Physiologically Relevant Validation: Utilizing advanced in vitro protocols designed to replicate physiological environments for reliable prediction of material performance and safety in vivo.
Meet the Experts

Marjan Marefat

Prof. Dr. Khatereh Kafshdozan
Offered Services
05 in vitro III (microiology)
• Disc diffusion
• OD and spread test
•And More