Myocardial infarction leads to irreversible loss of cardiomyocytes and formation of scar tissue that disrupts the heart’s electrical conductivity and contractility. Traditional therapies only manage symptoms but fail to restore native tissue architecture and function.
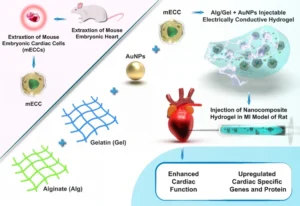
Tissue engineering approaches using electrically conductive hydrogels offer a promising solution by providing both structural support and electrical signaling to reestablish synchronized myocardial contraction. This study presents MRGel, an injectable hydrogel composed of gelatin, alginate, and gold nanoparticles (AuNPs), engineered to mimic the native cardiac microenvironment and support the growth and function of mouse embryonic cardiac cells (mECCs).
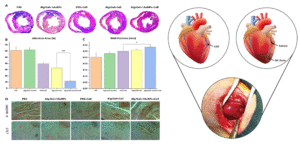
The MRGel demonstrated significant therapeutic potential in a rat model of myocardial infarction, where its injectability and electrically conductive composition enhanced the regeneration of damaged cardiac tissue. The uniform dispersion of AuNPs within the gelatin/alginate matrix facilitated improved electrical conductivity, promoting effective electrical coupling between transplanted mECCs. In vivo results showed enhanced mECC survival, alignment, and integration into host tissue, along with a marked reduction in fibrotic area and improved left ventricular function. Notably, MRGel exhibited excellent biocompatibility, with no significant inflammatory response, confirming its suitability as a minimally invasive, cell-supportive platform for cardiac tissue repair.
To access the paper, please click here.
