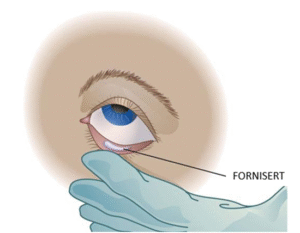
Key challenges in ocular drug delivery associated with conventional eye drops comprise poor drug retention, frequent dosing, and inconsistent therapeutic outcomes.
By providing sustained and localized release directly to the subconjunctival fornix, FORNISERT provides prolonged drug exposure within the therapeutic window, reduces the risk of toxicity or sub-therapeutic levels, and improves patient compliance. This innovative insert offers a minimally invasive solution with better drug retention and more reliable treatment for ocular inflammatory and immune-mediated conditions. FORNISERT is an innovative silicone-based ocular insert developed for the sustained and localized delivery of Cyclosporine A (CsA) directly to the subconjunctival fornix.
As illustrated in the following, the release curve demonstrates a gradual, controlled release of CsA over time (ca. 35 days), maintaining concentrations within the therapeutic window and avoiding peaks that may cause toxicity or sub-therapeutic exposure.
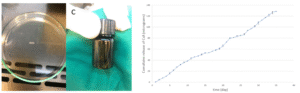
A pilot clinical trial for proof of concept demonstrated the effective placement and retention of FORNISERT in the conjunctival fornix of patients suffering from pterygium. This ensures effective CsA delivery and patient convenience by administering our FORNISERT.
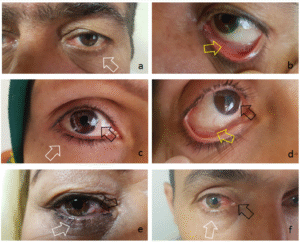
Key references depicted in the figure:
In Press, check back for updates.
