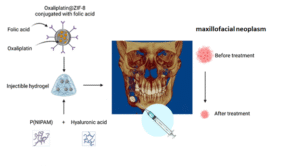
Bone cancer recurrence remains a significant challenge in cancer treatment. This project aims to develop a biocompatible and injectable hydrogel scaffold for localized drug delivery, aiming to prevent bone cancer recurrence after surgery.
In this project, a scaffold comprising a thermo-responsive poly(N-isopropyl acrylamide) (PNIPAAm) hydrogel, combined with hyaluronic acid (HA), is designed to enhance biocompatibility and provide a suitable environment for cell growth. The scaffold’s injectable nature allows for minimally invasive delivery, filling the surgical cavity and providing localized drug delivery. The hydrogel is further enhanced with folate-modified zeolitic imidazolate framework-8 (ZIF-8) nanoparticles loaded with Oxaliplatin, a potent chemotherapeutic agent. The folate modification targets the overexpressed folate receptors on bone cancer cells, enhancing drug delivery specifically to tumor cells and minimizing damage to healthy tissues. ZIF-8 nanoparticles are pH-responsive, degrading rapidly at the acidic pH of the tumor microenvironment, releasing Oxaliplatin locally and efficiently. This targeted drug delivery system aims to prevent tumor regrowth by eliminating residual cancer cells and reducing the risk of recurrence after bone cancer surgery. The innovative combination of biocompatible materials, targeted delivery, and pH-responsive drug release holds great potential for improving treatment outcomes and enhancing the quality of life for bone cancer patients.
